Advancing Precision Nutrition Through Comprehensive Glycomics
The carbohydrate components (glycans) of our diet are critical for human health, yet they remain the least structurally characterized macronutrient. Historically, the complexity of these molecules, ranging from simple sugars to large, intricate polysaccharides, has defied detailed analysis, leading to reliance on rudimentary classifications like “total carbohydrate” or “fiber”. This lack of molecular specificity severely limits progress in predicting efficacious fiber-based interventions and advancing the nascent field of precision nutrition.
Our laboratory develops and employs cutting-edge mass spectrometry (MS)-based glycomic platforms to overcome these analytical challenges, providing the molecular detail necessary to bridge the gap between food structure and health outcomes. We are focused on elucidating the complete chemical structures of dietary carbohydrates, recognizing that glycosylation plays a significant role in the structural and functional properties of biologically significant glycoproteins.
Our Multi-Glycomic Research Platform
We utilize a comprehensive, high-throughput, multi-glycomic workflow for characterizing the complex structural diversity found in food. This includes targeted platforms for all major carbohydrate types:
- Polysaccharide Identification (FITDOG): We utilize Fenton’s Initiation Towards Defined Oligosaccharide Groups (FITDOG)—a nonenzymatic chemical digestion process coupled with high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (HPLC-QTOF MS)—to generate unique oligosaccharide fingerprints that identify and provide absolute quantitation of major polysaccharides (e.g., starch, cellulose, $\beta$-glucan, xyloglucan, mannan) in complex food matrices.
- Glycosidic Linkage Analysis (LACS): Our Linkage Analysis of Carbohydrates (LACS) method employs ultrahigh-performance liquid chromatography coupled with triple quadrupole mass spectrometry (UHPLC/QqQ MS) in dynamic multiple reaction monitoring (dMRM) mode to profile the precise connections between sugar units. We have developed an extensive linkage library capable of simultaneously characterizing over 90 unique glycosidic linkages, representing terminal, linear, bisecting, and trisecting species.
- Monosaccharide Composition (MACS): We perform Monosaccharide Analysis of Carbohydrates (MACS) using high-throughput LC-MS analysis for the absolute quantitation of 14 naturally occurring monosaccharides.
- Alcohol Soluble Carbohydrates (CASCADE): The Combined Alcohol Soluble CArbohydrate DEtermination (CASCADE) platform provides rapid-throughput and absolute quantitation of low molecular weight sugars, including mono-, di-, and oligosaccharides (such as raffinose), which are critical components of both simple sugars and soluble fiber.
We also employ a multidimensional 3D LC-MS/MS workflow that integrates high-resolution QTOF sequencing with subsequent dedicated MACS and LACS analyses on fractionated samples to achieve de novo structural elucidation of complex oligosaccharides derived from polysaccharides.
Impact and Application
The data generated by our advanced methodology feeds the Davis Food Glycopedia—a comprehensive resource cataloging quantitative structural glycan features in hundreds of foods. This glycomic data is crucial for clinical studies, allowing us to:
- Link specific glycan features to health outcomes, demonstrating that specific molecular features (like xyloglucan or raffinose) are more important predictors of conditions like insulin resistance (IR) than traditional metrics like total fiber or total sugars.
- Guide the formulation of diets highly enriched for specific monosaccharide residues or fiber structures to precisely modulate the gut microbiome.
By generating this deep structural detail, we are providing the essential analytical toolbox needed to advance Precision Medicine and Nutrition and optimize dietary choices based on the glycan diversity, quantity, and quality of food.
Milk Glycomics
Human Milk Oligosaccharides (HMOs) are non-digestible sugars that benefit the health and development of infants (1,2). HMOs help certain beneficial bacteria proliferate in the infant’s intestines, suppressing the growth of some pathogenic bacteria (3). HMOs also interact with pathogenic bacteria, preventing the bacteria from binding to intestinal epithelial cells.
HMOs interact with bacteria by binding to the proteins on the surface of the bacterial cells. The binding to beneficial bacteria is the first step, followed by enzymatic hydrolysis of the glycosidic bonds and metabolism of the resulting monosaccharides by the bacteria. On the other hand, binding to pathogenic bacteria may be the only step in their interaction with pathogenic bacteria, blocking the bacteria from binding to the surface of the gut epithelial cells (4,5).
Unlike breast-fed infants, formula-fed infants develop a more adult-like intestinal microflora characterized by a lower percentage of beneficial bacteria (1,3). The adult-like microflora have been linked to reduced health of the immune system for infants and may be caused by the lack of HMOs in infant formula.
Although infant formulas contain non-digestible sugars (usually from plants), they are structurally and compositionally less complicated than HMOs (6). The non-digestible substitutes have not been altered to resemble HMOs because only a few characteristics are known that affect their interaction with the bacteria, such as size and fucosylation (5,7). Even after narrowing the category to small, fucosylated HMOs, the remaining HMOs can be categorized into groups such as branched vs. linear structures and Lewis type (a, b, x, or y). Some of these motifs may bind the bacteria more effectively than others.
Human milk also contains sugars conjugated to proteins and lipids. The role of the conjugated sugars is apparently different than the role of the free sugars (HMOs). Our goal to annotate all glycans of both free and conjugated forms and to investigate the timeliness of their biosynthesis as well as the specificity of their interaction with sugar binding molecules in the infant gut.
After creating a high throughput method, we can now quantify HMO profiles across a large batch of different populations, timepoints, and diseases .
Above: 10% ACN, 20% ACN, and 40% ACN in 0.05% TFA fractions of an HMO pool can be easily profiled using MALDI-FTICR-MS. In 10% ACN, more neutral structures are found, while in 20% ACN a mixture of neutral and acidic compounds elute, and finally in 40% ACN in 0.05% TFA, mainly acidic structures are found.
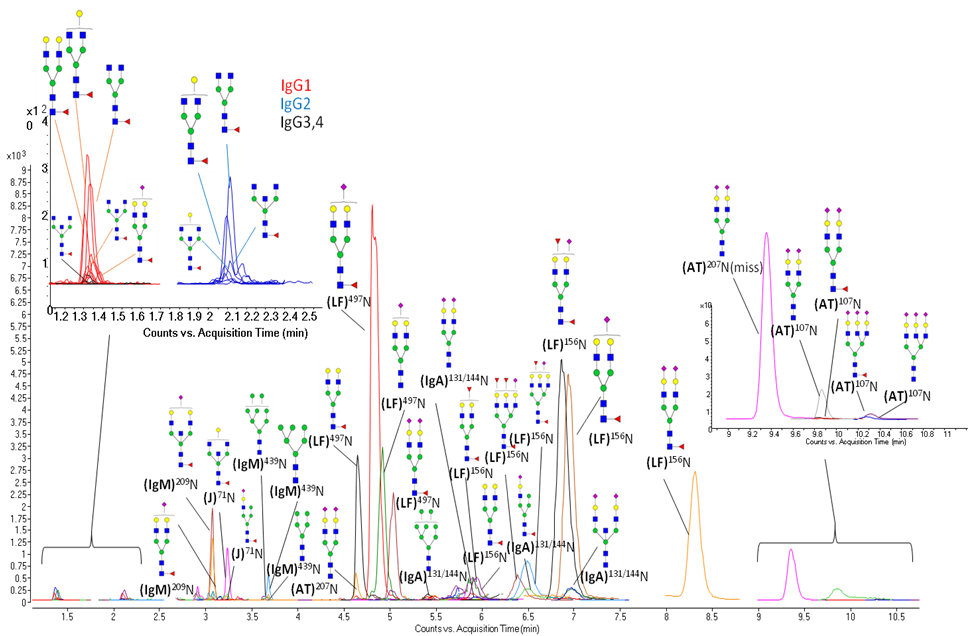
Milk Glycoproteomics
Proteins are also one of the major components in milk and a large subset of milk proteins are glycosylated. These glycoproteins make prominent contributions to the health benefits ascribed to consuming milk. Our research aims at establishing structure-specific functional benefits of the unique protein glycosylation profiles in milk.
To this end, strategies involving MS-based proteomics, gel-based quantitative protein profiling, and site specific glycosylation analysis are being used to establish the milk glycoproteome.
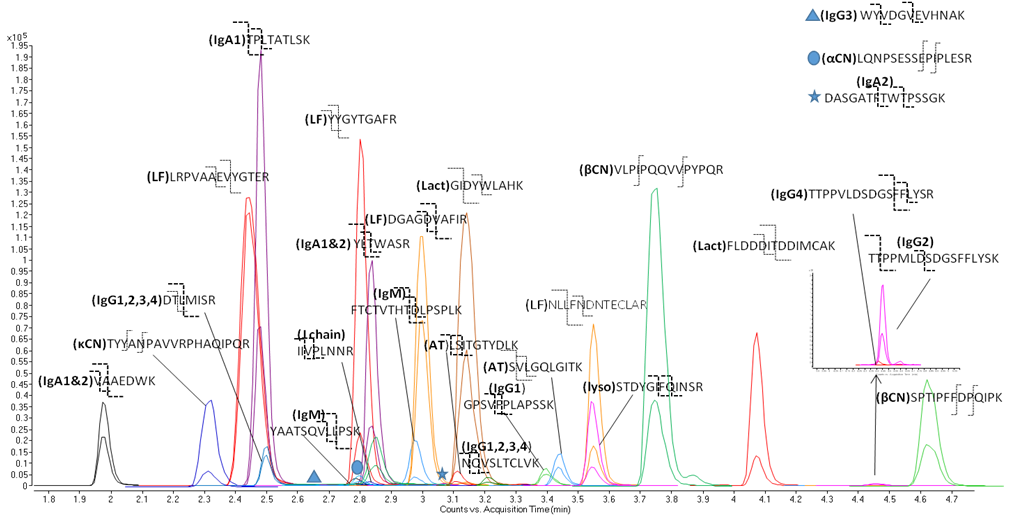
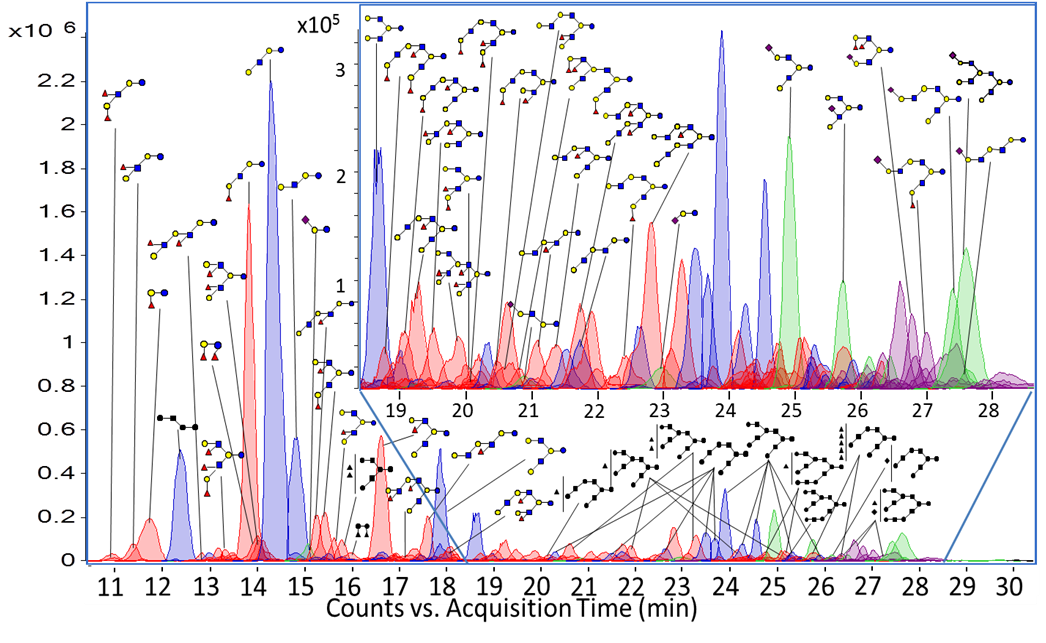
Milk Peptidomics
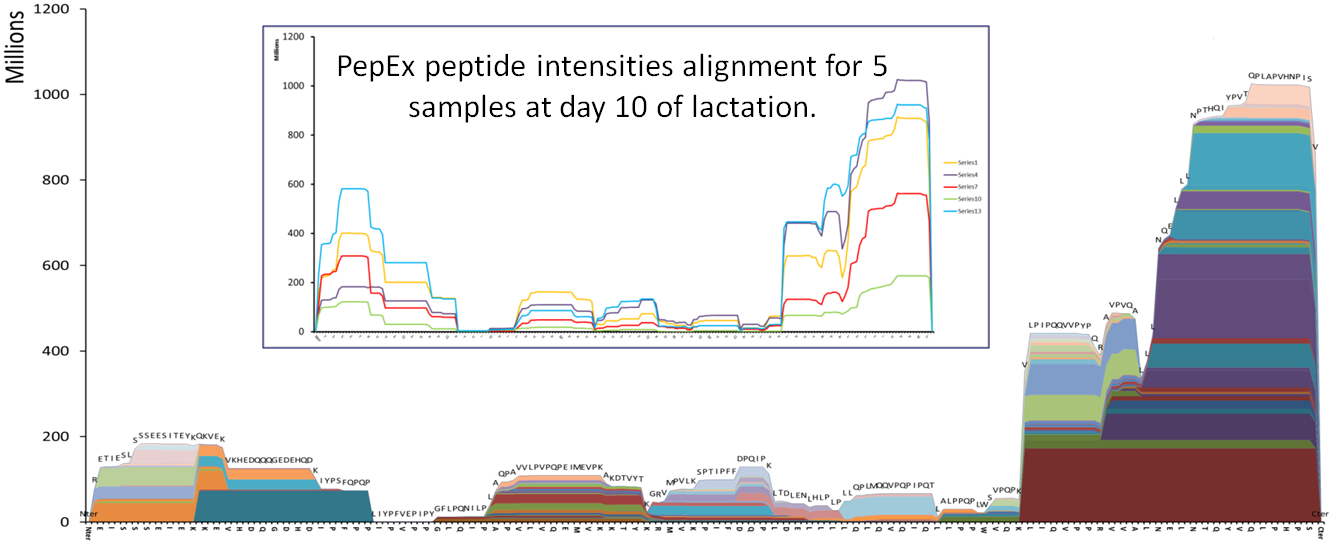
Milk is a self-digesting biofluid rich in proteolytic enzymes. A comprehensive study of the peptide content in human milk has revealed a vast diversity of naturally occurring peptides (NOPs). A human milk NOPs library has been created with more than 800 unique sequences from 34 proteins.
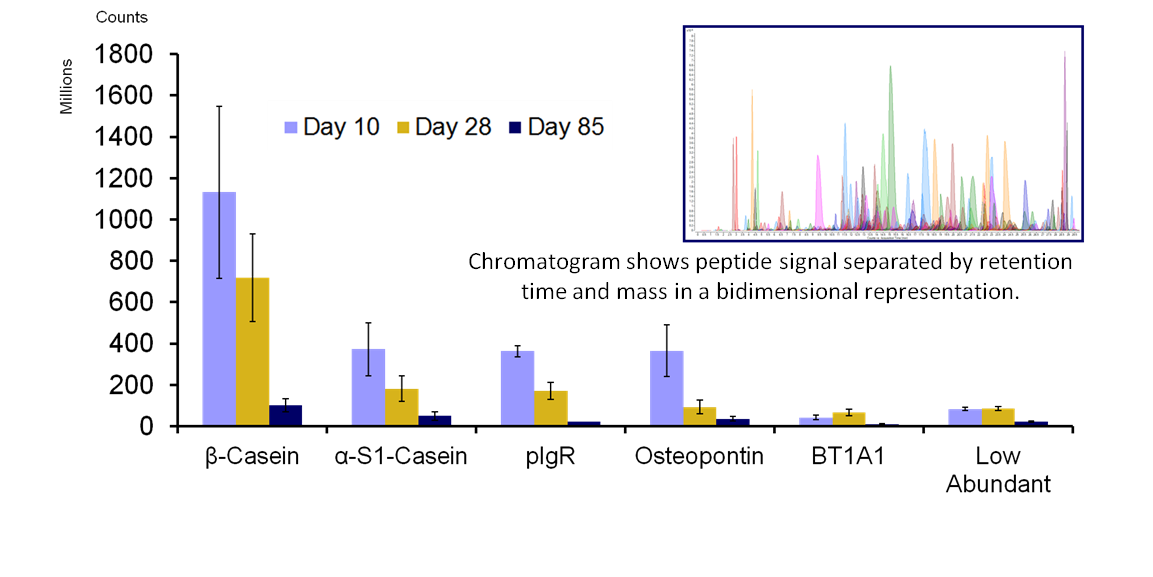
Human milk samples from 5 different mothers at three different timepoints of lactation were studied via HPLC Chip/Q-TOF. NOPs intensities were grouped, mapped and interpreted using an in-house software.
References
- Davis M.K. Breastfeeding in chronic disease in childhood and adolescence. Pediatr. Clin. North Am 2001, 48, 125-141.
- Boehm, Günther; Moro, Guido. Structural and Functional Aspects of Prebiotics Used in Infant Nutrition. J. Nutr. 2008, 138, 1818S-1828S.
- Harmsen H.J.; Wildeboer-Veloo A.C.; Raangs G.C.; Wagendorp A.A.; Klijn N.; Bindels J.; Welling G.W.. Analysis of intestinal flora development in breast fed and formula fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61-67
- Boehm, Günther; Moro, Guido. Structural and Functional Aspects of Prebiotics Used in Infant Nutrition. J. Nutr. 2008, 138, 1818S-1828S.
- LoCascio, Riccardo G.; Ninonuevo, Milady R.; Freeman, Smara L.; Sela, David A.; Grimm, Rudolf; Lebrilla, Carlito B.; Mills, David A.; German, J. Bruce. Glycoprofiling of Bifidobacterial Consumption of Human Milk Oligosaccharides Demonstrates Strain Specific, Preferential Consumption of Small Chain Glycans Secreted in Early Human Lactation. J. Agric. Food Chem. 2007, 55, 8914-8919.
- Boehm, Günther; Stahl, Bernd. Oligosaccharides from Milk. J. Nutr. 2007, 137, 847S-849S.
- Newburg D.S.; Ruiz-Palacios G.M.; Altaye M.; Chaturvedi P.; Meinzen-Derr J.; Guerrero M.D.; Morrow A.L.. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004, 14, 253-263.
- Dallas DC, Guerrero A, Parker EA, Robinson RC, Gan J, German JB, Barile D, Lebrilla CB (2015) Current peptidomics- Applications, purification, identification, quantification, and functional analysis. Proteomics 15 (5-6):1026-1038. doi:10.1002/pmic.201400310
- De Leoz ML, Kalanetra KM, Bokulich NA, Strum JS, Underwood MA, German JB, Mills DA, Lebrilla CB (2014) Human Milk Glycomics and Gut Microbial Genomics in Infant Feces Show a Correlation between Human Milk Oligosaccharides and Gut Microbiota- A Proof-of-Concept Study. Journal of proteome research. doi:10.1021/pr500759e
- De Leoz ML, Wu S, Strum JS, Ninonuevo MR, Gaerlan SC, Mirmiran M, German JB, Mills DA, Lebrilla CB, Underwood MA (2013) A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants.. Analytical and bioanalytical chemistry 405 (12):4089-4105. doi:10.1007/s00216-013-6817-1
- Guerrero A, Dallas DC, Contreras S, Bhandari A, Canovas A, Islas-Trejo A, Medrano JF, Parker EA, Wang M, Hettinga K, Chee S, German JB, Barile D, Lebrilla CB (2015) Peptidomic analysis of healthy and subclinically mastitic bovine milk. International dairy journal / published in association with the International Dairy Federation 46:46-52. doi:10.1016/j.idairyj.2014.09.006
- Hong Q, Ruhaak LR, Totten SM, Smilowitz JT, German JB, Lebrilla CB (2014) Label Free Absolute Quantitation of Oligosaccharides using Multiple Reaction Monitoring.. Analytical chemistry. doi:10.1021/ac404006z
- Huang J, Guerrero A, Parker E, Strum JS, Smilowitz JT, German JB, Lebrilla CB (2015) Site-specific Glycosylation of Secretory Immunoglobulin A from Human Colostrum. Journal of proteome research. doi:10.1021/pr500826q
- Newburg DS, Ruiz-Palacios GM, Morrow AL (2005) Human milk glycans protect infants against enteric pathogens. Annual review of nutrition 25:37-58. doi:10.1146/annurev.nutr.25.050304.092553
- Ruhaak LR, Lebrilla CB (2012) Analysis and role of oligosaccharides in milk.. BMB reports 45 (8):442-451. doi:10.5483/BMBRep.2012.45.8.161
- Ruhaak LR, Lebrilla CB (2012) Advances in analysis of human milk oligosaccharides.. Advances in nutrition 3 (3):406S-414S. doi:10.3945/an.112.001883
- Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL (2014) Breast Milk Oligosaccharides: Structure-Function Relationships in the Neonate. Annual review of nutrition 34:143-169. doi:10.1146/annurev-nutr-071813-105721
- Totten SM, Wu LD, Parker EA, Davis JC, Hua S, Stroble C, Ruhaak LR, Smilowitz JT, German JB, Lebrilla CB (2014) Rapid-throughput glycomics applied to human milk oligosaccharide profiling for large human studies. Analytical and bioanalytical chemistry 406 (30):7925-7935. doi:10.1007/s00216-014-8261-2
- Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB (2012) Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers.. Journal of proteome research 11 (12):6124-6133. doi:10.1021/pr300769g
- Wu S, Grimm R, German JB, Lebrilla CB (2011) Annotation and structural analysis of sialylated human milk oligosaccharides. Journal of proteome research 10 (2):856-868. doi:10.1021/pr101006u
- Wu S, Tao N, German JB, Grimm R, Lebrilla CB (2010) Development of an annotated library of neutral human milk oligosaccharides.. Journal of proteome research 9 (8):4138-4151. doi:10.1021/pr100362f